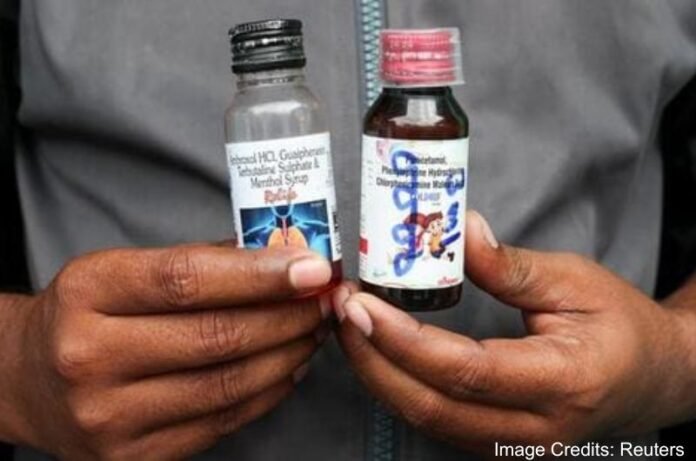
The World Health Organization (WHO) has issued a global alert warning against the use of three Indian-made cough syrups – Coldrif, Respifresh TR, and ReLife, after the deaths of several children in India, citing contamination with toxic diethylene glycol (DEG).
The UN health body said the alert followed confirmation from India’s Central Drugs Standard Control Organization (CDSCO) that the syrups contained dangerously high levels of DEG, a substance known to cause kidney failure and death when ingested.
According to WHO, CDSCO reported on October 8 that the tainted products, manufactured by Sresan Pharmaceutical, Rednex Pharmaceuticals, and Shape Pharma, were consumed by the affected children.
Tests found DEG concentrations nearly 500 times above the permissible limit – 48.6% compared to the allowed 0.1%.
Indian authorities have suspended manufacturing licenses and halted production at the implicated facilities, initiating product recalls across affected regions.
While CDSCO confirmed none of the syrups were exported, WHO urged global regulators to remain vigilant, especially in unregulated markets, and to monitor other oral liquid medicines made at the same sites since December 2024.